The bad news first--we're working on a record. So far today we're at a bit over 2,200 deaths from Covid19. That's a record, and it translates to a 15,400 weekly rate. That's a lotta dead people in a short time span. Do I know how long that will continue? Of course not. It's just a marker for now.
When will we reopen? That's what everybody wants to know, and that's part of what Dr. Scott Gottlieb talks about in a long interview at the liberal Vox:
Scott Gottlieb on how, and when, to end social distancing
The former FDA commissioner doesn’t think the US is going to return to normal anytime soon.
You can read it, FWIW. He's pessimistic, or at least that's how I would characterize his views.
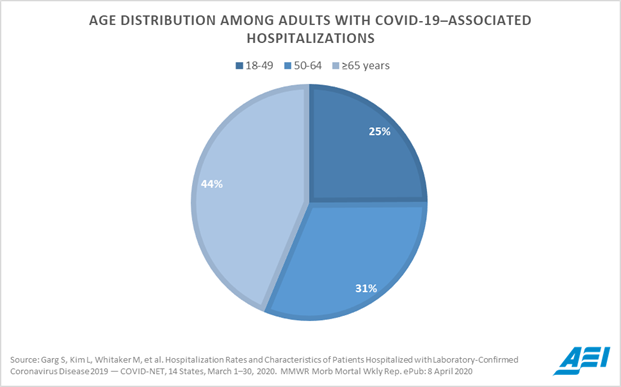
And here's a pie chart to provide reasons for being pessimistic. This thing isn't nearly so simple as many "conservatives" would like it to be, where you just tell oldies like me to go sit in a closet and everybody else can go out and be normal:
Please take note of the logo in the bottom right corner. AEI. This isn't data from WHO. It's not from China. It's from the American Enterprise Institute.
But on to the GOOD NEWS. Here's what Gottlieb is saying could happen while we wait for that vaccine--and please don't hold your breath for that. Nevertheless, what Gottlieb has to say in this excerpt from way down in the interview does hold out promise. Just forget about "herd immunity". Think about drug therapies, instead. Therapies that could be prophylactic--preventative--rather than just treating infections. That could really hold promise for getting back to a semblance of normality, and Gottlieb is optimistic about this. I believe he's referring to something I referenced in Interesting Interview Explaining Possible Antibody Treatments For Covid19--therapeutic antibodies:
Ezra Klein
Phase three, which is a much more normal phase of life, works off of the development of vaccines and therapeutics. Where do you think we are on that front and what do you think is plausible in terms of therapeutics, let’s say, by the end of the year?
Scott Gottlieb
We’re not going to have a vaccine by the end of the year. We need to assume that a vaccine may be two years away. So we need other technology. If you look at what could be available by the fall, it’s a small subset of drugs. I would be focusing attention and resources on working aggressively with those manufacturers to try to pull those products through the development process and understand whether they are safe and effective.
What are those drugs? One is an antiviral drug — it inhibits viral replication. And it’s pretty far along. There’s data available right now that suggests that it’s active. A lot of doctors are using it. The other products are therapeutic antibodies. These are basically biotech manufactured antibodies — the same kind of antibody your body would produce if it is exposed to the virus in order to fight the virus if you’re exposed again.
That’s an attractive product for a number of reasons: First, it can be used as a treatment early in the disease. It can also be used as a prophylaxis — as a bridge to a vaccine. You might be able to give a monthly injection or bi-monthly injection to people that would prevent them from getting infections. You can use that for frontline health care workers. You can use it for TSA agents or food handlers who are coming into contact with a lot of people and more likely get infected and then spread the infection. You can use it in the contacts of someone who is sick to prevent them from getting sick. That could be a very attractive drug.
The combination could be very effective if you can have both of those by the fall: a treatment to help people who are infected and an antibody that can help prevent infection in those who are exposed. That’s a pretty potent combination.
The biggest obstacle is going to be manufacturing them at scale. That’s something we could solve for right now. We should be working on how we’re going to make investments to help these companies scale up manufacturing and get to large commercial scale manufacturing in time for the fall so that if one of them does work, we’re able to turn on the spigot and produce millions of doses a month. That’s the kind of position you want to be in.
There was money set aside in a recent congressional bill that set aside upwards of $10 billion to do just this. That needs to be implemented. The companies need to be aware of it and pursue those opportunities. You need the agencies working to stand that up so that all has to happen. That’s the kind of thing I think we should be focused on right now.
Ezra Klein
In phase three you also talk about using serological surveys to determine population immunity. I’ve seen conflicting reports on whether people who’ve gotten coronavirus are immune. What is your best guess on that? If we had that serological testing, would it even matter?
Scott Gottlieb
Serological testing is important for understanding where the virus has been and who’s been exposed. It could be important for decisions about returning to work in certain professions where there’s gonna be high exposure like police officers or EMT workers or doctors and nurses. But by and large, what we’re going to find is that a very small percentage of the population has actually been exposed to this virus. If you talk to the modelers and the experts, they say anywhere from 1 to 5 percent of the US population has probably been exposed to this virus already.
If you look at the data coming out of Europe where they’re already using serology, it’s in the 5 percent range. So the idea that 30 or 40 percent of the population has had this virus is going to turn out not to be the case. It’s going to turn out to be a small percentage of the population — certainly in the single digits — that have the level of antibodies needed for immunity. And it’s not going to be enough to create herd immunity. It’s also not going to be enough to have this pool of people that can just return to work.
John Hinderaker at Powerline : https://www.powerlineblog.com/archives/2020/04/chloroquine-effectiveness-vs-coronaviruses-was-documented-in-2005.php
ReplyDeleteWe don't need to wait on a new drug. We have it now
I was just looking at my bookmarked twitter feeds. I came up with a link to this:
Deletehttps://www.judicialwatch.org/investigative-bulletin/trumps-winning-coronavirus-bet/
Yeah, I know Judicial Watch often hypes stuff, but I've been hearing similar reports. However, some of the therapies that Gottlieb is talking about do hold out great promise. The one about therapeutic antibodies? It works in like 20 minutes. And it can be used prophylactically. No side effects that I've heard of.
But yes, I do agree that HCQ needs to be widely available now, and if we can get some reports on its use beyond anecdotal stuff it should be part of the equation on reopenig.
I assume Trump is getting that info as soon as anybody. However, I just heard him saying we need to err on the side of caution.
I trust him.
I trust PDJT more than I trust Scott Gottleib. A not terribly impressive cousin of mine used to be a biggie with the AEI.
ReplyDeleteDr. Gottlieb was head of the FDA. He is also a partner in NEA (New Enterprise Associates), a very large (think megabucks) private venture capital firm.
ReplyDeleteFrom its Wiki page: NEA's investments include Formlabs, Masterclass, 23andMe, 3com, Appian, Bitglass, Bloom Energy, Box, Braintree, Boingo Wireless, Box, Buzzfeed, CareerBuilder, Caremark Rx, CCP Games, Climate Corporation, Cloudflare, Coursera, Cvent, Desktop Metal, Diapers.com, Drop, Duolingo, Enigma, Fetchr, Forter, Fusion-io, Groupon, Gilt Groupe, Global Savings Group, HealthSouth, Houzz, Jet.com, Juniper Networks, The Learning Company, Lot18, Macromedia, MapD Technologies, MongoDB, MuleSoft, Nicira, Opower, Pentaho, Raise Marketplace, Robinhood Markets, Salesforce.com, ScienceLogic, Semiconductor Manufacturing International Corporation, Smartcar, Snap Inc., Spreadtrum, Swiftype, Tableau Software, The Players' Tribune, Tempus, Threat Quotient, TiVo, Topera Medical, Toutiao, Uber, UUNET, Vonage, WebMD, Workday, ZeroFOX, and Zuoyebang.[16]
Any chance that Gottlieb might have a conflict of interest that might cause him to drag his feet re giving the afflicted hydroxycloroquine and/or the cocktail that includes it, curing people and getting our country back on track? Having known some very entrepreneurial doctors who aimed to do more than just take care of patients, I wonder...
Thanks for that. I have no particular brief for Gottlieb--in fact, none at all. I was wondering why no mention of HCQ. Distributed Bio isn't on that list, but others may be working on antibody therapy, too.
DeleteI'm hearing nothing but positive about HCQ. That said, a more specific antidote might be welcome. I'm sure Trump is on top of this and, if HCQ is as good as we're hearing, it will factor into his reopening calculations.
I believe he was talking about HCQ when he said "One is an antiviral drug — it inhibits viral replication. And it’s pretty far along. There’s data available right now that suggests that it’s active. A lot of doctors are using it."
DeleteAfter I posted that, I feared I was getting too paranoid. That’s what the internet can do to us sometimes… But I know that Scott Gottlieb was a Very Big Deal when he was at the FDA. I often wondered how many confused him with Michael Gottlieb, the physician who realized there was something connected and sinister about the unusual pneumonia and other diseases that were hitting gay men in San Francisco. He is given credit for being the first to identify acquired immune deficiency, which lead to the discover of HIV.
ReplyDeleteI believe, as you do, that our President listens to what these advisers have to say, processes it, then makes his decision. It is clear that he is taking this decision very seriously… He’s a far more serious thinker than his detractors would have us believe...
"Sometimes paranoia's just having all the facts."
DeleteWilliam S. Burroughs
Tom S
And no mention of masks. We should see there impact statistically soon.
ReplyDeleteAs well as more info on what treatments work.
And a guess / swag, I think we will see 10x current testing soon.
Mark, I really enjoy your blog and think you're a very bright guy. But I have to ask you, does it not even cross your mind that the daily corona death totals might be suspect and highly inflated. Are you not at all curious as to how to explain the drastic drop in daily deaths reported for the flu, diabetes, and heart disease. If not, perhaps you should take a look at it. There is an easy explanation that is publicly known. Coroners and medical officials around the country have been told to classify any death that might remotely have something to do with the virus as a fatality caused by the virus. If it looks like a fish, and smells like a fish, it probably is. Something is mighty "fishy" about the corona death rates and I would hope you come to recognize it. Blessings.
ReplyDeleteActually, I have addressed tht issue.
DeleteIndeed you have. But there may be a bit more jiggery-pokery going on than you imagine, now that we have vested interests.
DeleteThere is a greater than baby step chance we overreacted. Do you think anyone in government would ever admit that?
"there may be"
Delete"imagine"
"greater than baby step chance"
You're not exactly going out on a limb. Do you really expect Trump to act on such surmises?
He already has, actually, in going with the worst case models.
DeleteI was trying to be equivocal, because I really don't know. If I were to be firm, then yes. We overreacted. This virus may well be more than the flu, but it's not Ebola.
SARS-CoV-19 is actually FAR WORSE than Ebola, as a pandemic threat. Ebola is unquestionably many times more deadly on a case by case basis, but SARS-CoV-19 has a far greater propensity than Ebola to spread far and wide--and so has already killed far more people than Ebola has, or probably ever will. Ebola has only ever been a regional epidemic threat. That's what SARS was supposed to be, too. COVID took the scientific world by surprise by turning out to be a true pandemic threat.
DeleteBut there are also other viruses out there that could pose an even greater threat than COVID--bird flus that have jumped to humans, but still don't spread human to human. We're only one mutation away from unimaginable catastrophe, because those bird flus have proven far deadlier in the humans that have been infected than any other flu. Like up to 60%. That's what epidemiologists fear far more than coronaviruses.
Probably about 8% of the US population has been infected so far. Using Steve Sailors x 1,0000 x death rate. Rate of infections, even with the lockdowns, seems to be increasing.
ReplyDeleteFrustrating Areas:
- Overall US Positives for tests out of 146,000 daily tests is 20%
- No explanation on why the death rate is where it is in the US. It appears to be around 2,000 per day.
- No hard information on how Quinine does to treat, and it's status on being used to treat
- Lack of information on what is being done to increase testing
- No good information on impact of masks in the US. My guess is this will help.
- Biased hit pieces, such as one on South Dakota.
- No good information on what Taiwan has done to stay in business. They implemented 120+ measures, it would be nice to know what they are, and what can be copied in the US?
- Information on what is working for treatments, and what is not.
- Information on what is working to prevent infections, and what is not. Especially at a personal level.
The lack of transparency continues to be disturbing.
Deleteremdesivir is the new antiviral, from Gilead, that is in clinical trials in various parts of the world. This article is fresh - published yesterday - and covers remdesivir and hydroxychloroquine, along with several other drugs.
ReplyDeleteRemdesivir was recently given on a compassionate-use basis to 53 critically ill patients hospitalized in the U.S., Europe, Canada and Japan. Clinical improvement was observed in 68% of the patients treated, according to an analysis published in The New England Journal of Medicine. Each patient was given at least one dose of remdesivir and evaluated during a median follow-up of 18 days. According to the study results, 36 patients (68%) had an improvement in oxygen-support class, including 17 of 30 patients (57%) receiving mechanical ventilation who were extubated. A total of 25 patients (47%) were discharged, and 7 patients (13%) died; mortality was 18% (6 of 34) among patients receiving invasive ventilation and 5% (1 of 19) among those not receiving invasive ventilation.
“In studying remdesivir, the question is not just whether it is safe and effective against COVID-19 but in which patients it shows activity, how long should they receive treatment and at what stage of their disease would treatment be most beneficial,” wrote Daniel O’Day, CEO of Gilead, the pharmaceutical manufacturer of remdesivir, in an open letter last week. “Many answers are needed, which is why we need multiple types of studies involving many types of patients. Some of these answers will start to emerge in the coming weeks as we receive the first data from the various clinical trials underway.”
Worldwide, there are seven clinical trials underway to determine if remdesivir is safe and effective against COVID-19. Gilead says it expects to share preliminary data from a study of remdesivir in severe patients at the end of April, and will work quickly to interpret the findings. Come May, initial data from a placebo-controlled NIAID trial, as well as data from a Gilead study of patients with moderate symptoms of COVID-19, will also be available.
Much more here:
https://www.laboratoryequipment.com/563201-COVID-19-Treatment-Update-Remdesivir-Hydroxychloroquine-Leronlimab-Ivermectin-and-More/
Bebe, that link seems suspect to me. The comments about hydroxy quotes a chinese study and doesn't mention the excellent study by French virologist Dr Raoul. Looks like it's intended to discredit the drug.
DeleteThis is what the article says:
DeleteThus far, study results for hydroxychloroquine have been inconclusive. For example, results from the first controlled study of hydroxychloroquine for treating COVID-19 showed no significant difference in outcomes between those who received the drug and those who received standard care. The study, led by a team at the Shanghai Public Health Clinical Center in China, involved 30 patients hospitalized with confirmed COVID-19 between February 6 and February 25. Half the patients were randomly assigned to receive 400 mg of hydroxychloroquine per day for 5 days in addition to standard care, while the other patients in the control group received just standard care. There was no placebo. The researchers concluded that disease progression was statistically similar, although there was evidence of a reduction in viral load.
On the contrary, initial results from a placebo-controlled trial of hydroxychloroquine at Renmin Hospital of Wuhan University in Wuhan, China indicate that patients hospitalized with mild COVID-19 recovered more quickly with addition of the drug than with placebo at the start of a standard treatment. In this trial, 62 patients at the hospital were randomized to receive either a placebo or 200 mg of hydroxychloroquine twice daily for five days, in addition to standard care for both groups. According to the results, the 31 patients given hydroxychloroquine reported a normal body temperature and cessation of cough much quicker when compared with the 31 patients given the placebo. A larger proportion of patients on hydroxychloroquine also demonstrated an improved chest CT, with 61% showing “significant improvement.”
Seems pretty evenhanded to me. The results of the second study seem quite positive. And we have heard what it says: There is as yet no conclusive study that proves hydroxychloroquine should be the drug of choice for COVID-19.
I would not discount all studies done in China. Dismiss them out of hand. And I would not call this link “suspect”. I saw no intention to discredit the drug.
On Dr. Raoult’s latest study:
DeleteDr. Didier Raoult of Marseilles and his co-workers have published another preprint on clinical results with the chloroquine/azithromycin combination that their earlier work has made famous. And I still don’t know what to think of it.
This is going to be a long post on the whole issue, so if you don’t feel like reading the whole thing, here’s the summary: these new results are still not from randomized patients and still do not have any sort of control group for comparison. The sample is larger, but it’s still not possible to judge what’s going on. And on further reading, I have doubts about Dr. Raoult’s general approach to science and doubts about Dr. Raoult himself. Despite this second publication, I am actually less hopeful than I was before. Now the details.
Much more here:
https://blogs.sciencemag.org/pipeline/archives/2020/03/29/more-on-cloroquine-azithromycin-and-on-dr-raoult
Some are talking about chloroquinolone and hydroxycloroquinolone as though they were the same drug. They are not. Chloroquinolone can be toxic to some. Hydroxycloroquinolone may not be quite as effective. That remains to be seen. To read more about this:
Deletehttps://www.nature.com/articles/s41421-020-0156-0
It seems pretty clear that most of the studies on these drugs have occurred in China.
The sciencemag blogger said he didn't believe the HCQ results because they were too good to believe, and not as well documented as he'd like. The "too good to believe" results agree with reports from American doctors (and patients) I've read.
DeleteIt sounds as though Dr. Raoult’s work was not done in a truly scientific manner. Thus that makes it “anecdotal”. I would say that, although the latter can be compelling, our testing of the use of medicines for new applications usually follows the protocols used in the former. No one is saying Raoult is wrong.
ReplyDeleteAs for the sciencemag blogger, here is Derek Lowe. I try not to be condescending when it comes to bloggers:
An Arkansan by birth, Derek got his BA from Hendrix College and his PhD in organic chemistry from Duke before spending time in Germany on a Humboldt Fellowship on his post-doc. He’s worked for several major pharmaceutical companies since 1989 on drug discovery projects against schizophrenia, Alzheimer’s, diabetes, osteoporosis and other diseases.
Derek writes the popular blog In the pipeline on drug discovery and the pharma industry.
So why does Stephen Smith, a prominent virologist from NJ, back up everything Dr Raoult said ? Too many others, especially the chinese, have an agenda.
ReplyDeletehttps://www.foxnews.com/media/dr-stephen-smith-on-effectiveness-of-hydroxychloroquine-with-coronavirus-symptoms-beginning-of-the-end-of-the-pandemic